Many widely used environmental chemicals have been associated with adverse health effects, even when they do not directly target human cells.
Glyphosate is a clear example: although its biochemical target (the shikimate pathway) is absent in humans, it is present in many gut bacteria. This means that the gut microbiome, not human tissue—is the primary site of glyphosate action. In collaboration with Dr. Hans Lehmler, we demonstrated that chronic low-dose glyphosate exposure alters gut microbial communities, reducing beneficial commensals such as Lactobacillus and Bifidobacterium, while promoting bacteria associated with inflammatory signaling. These microbiome shifts are accompanied by increased colonic Th17 (CD4⁺ IL-17A⁺) responses and elevated Lipocalin-2, a marker of intestinal inflammation. Together, these findings show that glyphosate can drive immune dysregulation indirectly by reshaping the gut microbiota.
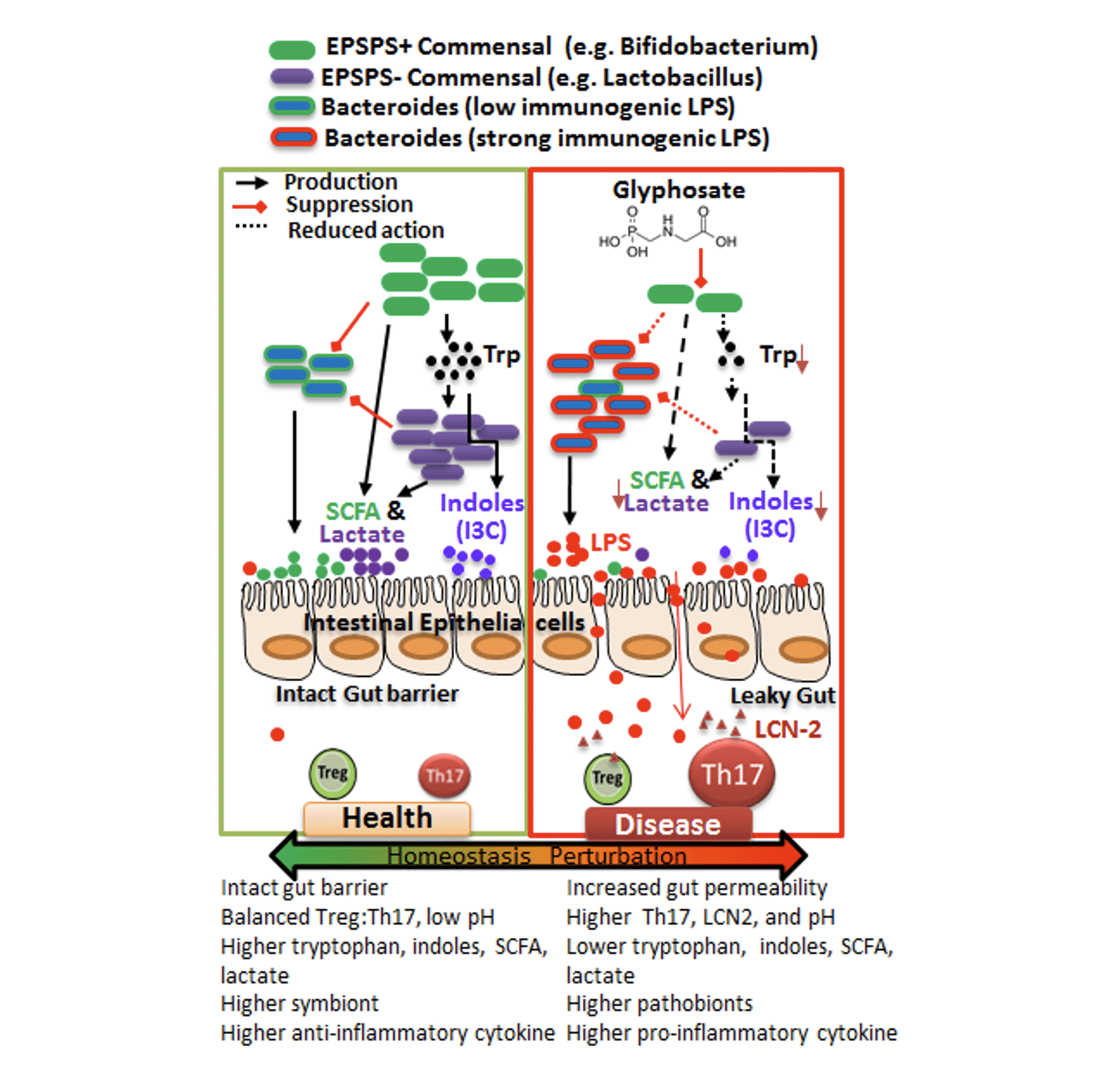
Key Findings & Interpretation
- Glyphosate does not act directly on human cells, instead, it targets microbial metabolic pathways in the gut.
- Even low-dose exposure results in loss of beneficial bacteria and expansion of inflammatory microbial networks.
- These microbiome changes correlate with enhanced Th17-driven mucosal inflammation and elevated gut inflammatory markers.
- The microbiome provides the mechanistic connection between environmental exposure and immune imbalance.
Future Directions
- Determine mechanism through which glyphosate induce depletion of beneficial bacteria such as Lactobacillus, Bifidobacterium etc. and enrichment of pathobionts.
- Test whether microbiome restoration strategies (e.g., synbiotics, targeted probiotics, diet-microbe pairing) can prevent or reverse glyphosate-induced dysbiosis and inflammation.
- Integrate multi-omics analyses (metagenomics, metabolomics, immunophenotyping) to identify microbial or metabolic biomarkers of glyphosate exposure risk.
- In collaboration with Dr. Christina Camell (University of Minnesota) and Dr. Hans Lehmler, investigate whether glyphosate-induced microbiome disruption contributes to chronic inflammatory states, including adipose inflammation, metabolic syndrome, and obesity, establishing whether microbiome-mediated inflammation links environmental exposure to metabolic disease risk.
Selected Publications
Simonsen D, Cady N, Zhang C, Shrode RL, McCormick M, Spitz DR, Chimenti MS, Wang K, Mangalam A, Lehmler HJ. The Effects of Benoxacor on the Liver and Gut Microbiome of C57BL/6 Mice. Toxicol Sci. 2022 Feb 28;186(1):102-117, PMID: 34850242

Lehman PC, Cady N, Ghimire S, Shahi SK, Shrode RL, Lehmler HJ, Mangalam AK. Low-dose glyphosate exposure alters gut microbiota composition and modulates gut homeostasis. Environ Toxicol Pharmacol. 2023;100:104149. doi: 10.1016/j.etap.2023.104149. PMID: 37196884
Dean LE, Wang H, Bullert AJ, Wang H, Adamcakova-Dodd A, Mangalam AK, Thorne PS, Ankrum JA, Klingelhutz AJ, Lehmler HJ. Inhalation of 2,2',5,5'-tetrachlorobiphenyl (PCB52) causes changes to the gut microbiome throughout the gastrointestinal tract. J Hazard Mater. 2024:480:135999. PMID: 39369679
Dean LE, Wang H, Li X, Fitzjerrells RL, Valenzuela AE, Neier K, LaSalle JM, Mangalam AK, Lein PJ, Lehmler HJ. Identification of polychlorinated biphenyls (PCBs) and PCB metabolites associated with changes in the gut microbiome of female mice exposed to an environmental PCB mixture. J Hazard Mater. 2025;489:137688. doi: 10.1016/j.jhazmat.2025.137688. PMID: 40020572